Write the importance of neutralization reaction.
1 Answer
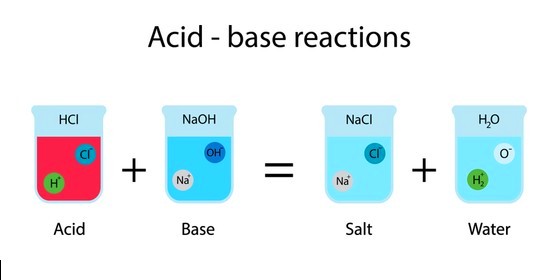
A neutralization reaction is where acid reacts with a base to form H2O and salt which are both neutral substances. Hydrogen ions from acid combine with hydroxide ions from the base to form water. The Neutralization Reaction can be written as follows:
Acid + Base → H2O + Salt
H+CL (Acid) + NaOH- (base) → H2O + NaCl (salt)
The importance of neutralization reactions in daily life are as follows:
- Indigestion:
Too much acid is produced inside the stomach during indigestion which results in stomach disorder or acidity. We take bases such as magnesium milk which contains magnesium hydroxide to retrieve the pain. Taking a base neutralizes the excess acid effect.
- Ant Sting: The ant sting can be painful as it has formic acid. We can neutralize this acid effect and relieve the pain caused by the sting by using moist baking soda which is basic in nature.
- Tooth Decay: When we eat food, the food molecules get decomposed by the action of the microorganisms present in the mouth. This further results in the formation of acid. This acid is majorly responsible for tooth decay. Toothpaste is generally made by alkaline substances to make its nature basic. So, when you brush your teeth, this basic nature of the toothpaste reacts with the acid produced by the microorganisms, which causes tooth decay. As a result, the harmful effect of the acid is neutralized, and it stops tooth decay.
- Shiny Hair Due to Conditioner: Our hair is rough after a shampoo, but it gets shiny after using a conditioner. This happens because shampoo is basic in nature, and the conditioner is acidic. So, the conditioner neutralizes its effect, and the hair becomes shiny.
- Monitor pH of the Soil: Plants grow on soil that has a particular pH value. Plants cannot grow on soil that has an acidic composition. So, to neutralize its effect bases are added to the soil. Compounds such as limestone, powdered lime, and also burnt wood ashes are added to the soil to make its pH less acidic. This technique helps in controlling the pH of the soil by neutralizing the effect of acids and bases in the soil.
-
How would you predict the geometry of the ammonia molecule on the basis of VSEPR theory? 1
-
What kind of hybridization results in trigonal planer geometry? 1
-
Which hybridization results in a linear geometry. Give an example. 1
-
What kind of hybridization results in tetrahedral geometry? Give an example. 1
-
What is the mode of hybridization for carbon in ethyne and oxygen in water? 1
-
Predict the mode of hybridization of B in BF3. write two features. 1
-
State any two proper conditions and mode of hybridization of C in C2H2? 1